Research in the Farmer Lab
Biocoordination of HNO
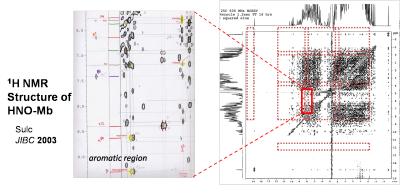
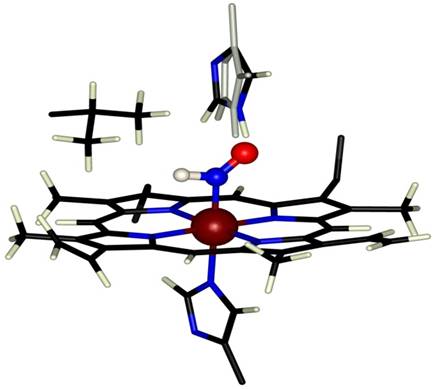
A long-running project in our lab concerns heme-based catalysis, focusing on the reactivity of metal-bound NOx intermediates. We have reported extensively on the stable HNO adduct of deoxymyoglobin, HNO-Mb, generated by reduction of nitrosyl myoglobin or by the trapping of free HNO by deoxyMb, characterized by NMR, resonance Raman and X-ray absorption spectroscopies. Recent work has demonstrated that stable HNO-adducts of other oxygen-binding globins may be generated by trapping of free HNO. The unique diamagnetic H NMR signal of the bound HNO allows us to interrogate the heme pocket of these species using 2D methods such as NOESY and HSQC. As HNO is isoelectronic with O2 and is efficiently trapped by O2-binding proteins, we hypothesize that HNO may inhibit or turnover oxygenases in the absence of O2. We propose that substitution of HNO for O2 may allow a unique approach to studying the mechanism of non-heme oxygenases.
ROS/RNS/RSS in Oxidative Stress
Oxidative stress is typically described as an effect driven by ROS, reactive oxygen species. But in the two decade the importance of reactive nitrogen and sulfur species, termed RNS and RSS, in human disease has been dramatically demonstrated. RNS, termed nitrosative stress, derives from the generation and consumption of NO, an amazingly ubiquitous signalling agent . Like NO, H2S is a toxic but biologically important molecule, bot appear to affect a wide spectrum of biological activities such as vasorelaxation, neuromodulation and inflammation. This diversity of functions may result from the range of species that derive from H2S and NO by poorly defined redox reactivity, likely yielding novel species whose presence and physiological importance remains unknown. We several have projects concerning RNS, such as models for Nitric Oxide Dioxygenase activity, and RSS, to characterize polysulfane and other products formed from reductive reactions of nitrosothiols with H2S and other reductants; to interrogate the reaction pathways using a combination of high resolution mass spectroscopy, isotopic labelling and chemical probes.
Flavonol Complexes
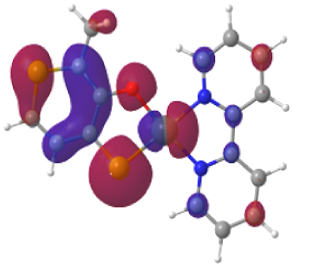 Light-to-chemical-energy conversion is an energy resource that is both clean and renewable. Many photovoltaic systems convert photon energy to long-lived excited state species, trapping light energy in chemical form. Less studied are those in which the excited state is truly dark, i.e. non-emitting. We are investigating a family of heterocyclic dyes with “pre-aromatic” constructs, in that oxidation generates pseudo-aromatic tautomers. These dyes are structurally related to the naturally occurring flavonals, which are widely studied antioxidants found in the skin of fruit and berries. The metal complexes of these dyes have unusual photoreactivity. For example, the Pt(II)(bpy)(dye) complexes of the N, S, O-heterocycles are strong photoreductants with > microsecond emission lifetimes at room temperature. We hope to explore the application of these heterocyclic dyes and their metal complexes in dye-sensitized solar cells (the so-called Gratzel cells).
Light-to-chemical-energy conversion is an energy resource that is both clean and renewable. Many photovoltaic systems convert photon energy to long-lived excited state species, trapping light energy in chemical form. Less studied are those in which the excited state is truly dark, i.e. non-emitting. We are investigating a family of heterocyclic dyes with “pre-aromatic” constructs, in that oxidation generates pseudo-aromatic tautomers. These dyes are structurally related to the naturally occurring flavonals, which are widely studied antioxidants found in the skin of fruit and berries. The metal complexes of these dyes have unusual photoreactivity. For example, the Pt(II)(bpy)(dye) complexes of the N, S, O-heterocycles are strong photoreductants with > microsecond emission lifetimes at room temperature. We hope to explore the application of these heterocyclic dyes and their metal complexes in dye-sensitized solar cells (the so-called Gratzel cells).
Melanin/Melanom a
a
We are interested in the coordination and redox chemistry of the black pigment melanin, the color in your hair and skin, especially as it relates to melanoma cancer. Melanins consist of small sheets of oligomeric dihydroxyindoles (derived from oxidation of dopamine), which aggregate by pi-stacking and hydrogen bonding into small nanoparticles as shown. Metal-complexation by melanin promotes its redox cycling, and we hypothesized that this pro-oxidant response may be cytotoxic to melanoma. Along these lines, we are investigating metal-based drug therapies for melanoma, designing new sulfur-based chelators that have shown toxicity against melanoma. This has also engendered new fundamental studies of oxidative desulfurization reactions of these metal complexes, a reactivity that we believe may be central to their cytotoxic effects. We have also use synthetic melanin nanoparticles and extracted melanins from natural sources to investigate the photochemical and redox reactivity of the aggregates.